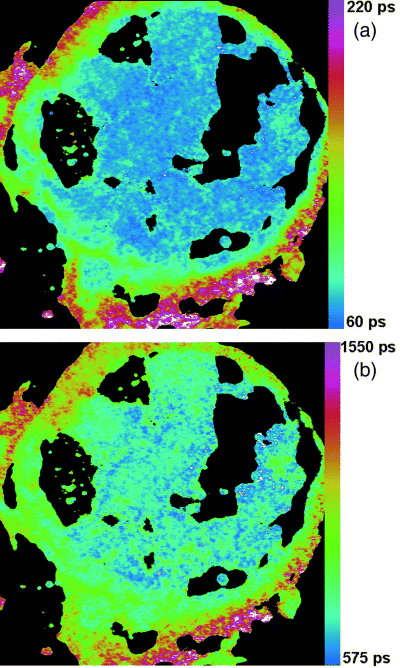
Fluorescence Lifetime Imaging (FLIM)
Fluorescence Lifetimes
It’s not something we normally consider, but fluorescence takes time—once a fluorophore absorbs a photon, it holds on to the energy for a while before emitting it. If you shine a very short pulse of light on a fluorescent sample, the intensity of the emitted light decays exponentially with time, and the time constant for this decay is called the Fluorescence Lifetime. The cool thing about the fluorescence lifetime is that you can use it to investigate the environment of the fluorophore.
Fluorescence Quenching and FRET
Many fluorophores respond to their environment. E.g, in Fluorescence Resonance Energy Transfer (FRET) you excite one fluorophore (the donor) which, rather than emitting the light, gives its energy to an acceptor fluorophore if it is nearby, which emits instead. Because the fluorophores need to be close together, it allows us to measure the proximity of molecules e.g. proteins in cells. Other substances in the sample can also act as acceptors, and if they are not fluorophores we call it quenching.
Without putting in an acceptor fluorophore, just looking at the intensity doesn’t tell us about the quenching—a decrease in intensity could simply be photobleaching or decreased amount of fluorophore. The great thing about the fluorescence lifetime imaging is that it gives us two parameters, the amplitude of the exponent (the amount of fluorophore present) and the exponential decay constant (which corresponds to the degree of quenching).
Developing a Fluorescence Lifetime Imaging (FLIM) system.
My first venture into microscopy involved developing a fluorescence lifetime imaging system. I was in Paul French’s lab, working with the ultrafast lasers needed to produce the very short pulses to excite the fluorophores. We took the data with a time-gated image intensifier, like an ordinary microchannel-plate image intensifier, but with a very fast ‘shutter’ that allowed us to take an image of the emitted light at a specific time just after the excitation pulse.
I developed the delay-line control, imaging capture and analysis sides of the project—writing code to control the equipment that managed the shutter delay, take a series of pictures with different delays, then fit single (or multiple) exponentials, pixel-by-pixel to this time-series data. To calculate the fluorescent lifetime images, I iteratively least-squares fit each pixel of a low-res scan of the image, then used this low-res image to seed the start values for iteratively fitting nearby pixels in the high-res image. This seeding method reduces the number of iterations and makes the code very quick—I even managed to get it working in real-time (which back then was quite a challenge)!
An Extra Dimension of Information
This means that we can see an extra dimension of information—the fluorescence lifetime—in our microscope images. Fluorophores now not only have a signature absorption and emission spectrum, but a characteristic lifetime, which, depending on the fluorophore chosen, can change in response to the environment, giving us a extra measure of what’s going on our sample.
References
High-resolution whole field fluorescence lifetime imaging of fluorophore distribution and environment Dayel, MJ.; Dowling, K; Hyde, S. C.; Dainty, J.C.; French, P.M.W.; Vourdas, P.; Lever, M. J.; Dymoke-Bradshaw, Ay K.; Hares, J D.; Kellett, P.A. Proc. SPIE Vol. 3196, pp 111-117, 1998.Optical and Imaging Techniques for Biomonitoring III
Time-domain whole-field fluorescence lifetime imaging with optical sectioning. Cole MJ, Siegel J, Webb SE, Jones R, Dowling K, Dayel MJ, Parsons-Karavassilis D, French PM, Lever MJ, Sucharov LO, Neil MA, Juskaitis R, Wilson T. Journal of Microscopy. 2001 Sep;203(Pt 3):246-57.
High resolution time-domain fluorescence lifetime imaging for biomedical applications. Dowling, K; Dayel, MJ; Hyde, SCW; French, PMW; Lever, MJ; Hares, JD; Dymoke-Bradshaw, AKL. Journal of Modern Optics, 1999, Vol 46, No 2, pp 199-209.
Whole-field fluorescence lifetime imaging with picosecond resolution using ultrafast 10-kHz solid-state amplifier technology. Dowling K.; Dayel M.J.; Hyde S.C.W.; Dainty J.C.; French P.M.W.; Vourdas P.; Lever M.J.; DymokeBradshaw A.K.L.; Hares J.D.; Kellett P.A. IEEE Journal of Selected Topics in Quantum Electronics, 1998, Vol.4, No.2, pp 370-375
Fluorescence lifetime imaging with picosecond resolution for biomedical applications. Dowling K.; Dayel M.J.; Lever M.J.; French P.M.W.; Hares J.D.; DymokeBradshaw A.K.L. Optics Letters, 1998, Vol.23, No.10, pp 810-812
Two-dimensional fluorescence lifetime imaging for in-vitro and in-vivo application. French, Paul M.; Dayel, MJ.; Dowling, K; Hyde, S. C.; Lever, M. J.; Vourdas, P.; Dymoke-Bradshaw, A K.; Hares, J D. Proc. SPIE Vol. 3250, pp 150-157, 1998. Optical Biopsy II